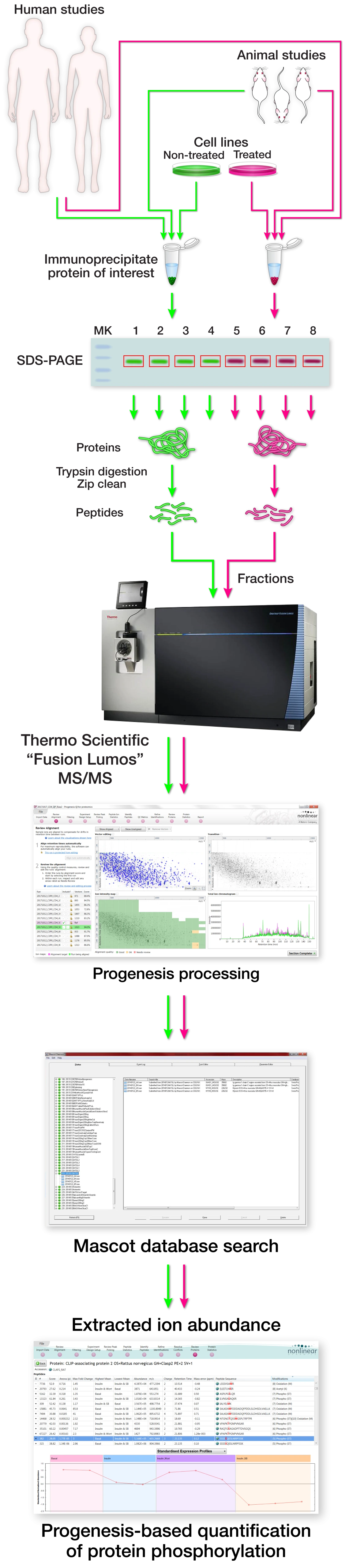
Identifying post-translational modifications (PTMs) and quantifying changes in PTM abundance after cell treatments (like kinase inhibitors for example), across either cell or animal models of disease states as well as human control vs disease state tissue.
If you have a protein of interest and would like to identify post-translational modifications and quantify effects of cell treatment on directional abundance changes of the PTMs, we would like to help. It is our responsibility to aid in the design and execution of the experiment. It begins with a conversation of the specifics and discussion of feasibility, potential complications, and hopeful outcomes. After that, due diligence is performed to ensure efficient "target" protein purification and yeild. Once appropriate target protein has been validated a decision is made on the final experimental design and cost. After that, protein lysates from cell culture or animal/human tissue are subjected to target protein immunoprecipitation. The immunoprecipitates are then separated by SDS-PAGE and the size of the target protein is then excised as a gel slice. Each gel slice is then prepped, subjected to enzymatic digestion to digest the protein into peptides and the resultant peptides are then desalted. Each gel slice's purified peptides are then analyzed as independent samples by mass spectrometry. The mass spec raw data is then processed in the quantitative proteomics software program Progenesis followed by searching against the appropriate database with the software program Mascot. The Mascot peptide and protein identification data is then imported into Progenesis. The proteins are then analyzed for PTM abundance changes across the different treatment groups.