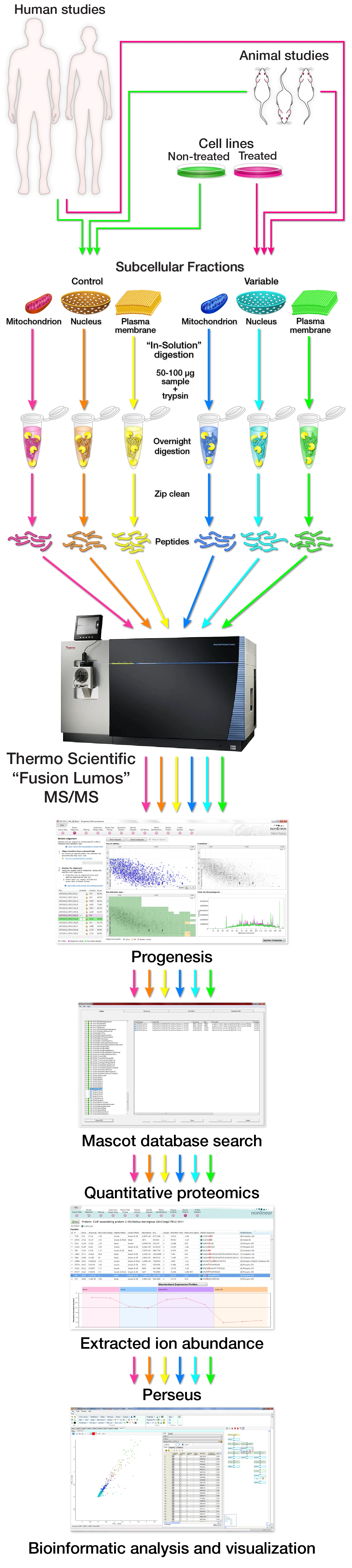
Profiling protein abundance changes across subcellular fractions, from either cell culture systems, animal models, or human studies.
If you have a hypothesis that a certain biological perturbation results in protein expression changes within a particular subcellular organelle, we would like to help. It is our responsibility to aid in the design and execution of the experiment. It begins with a conversation of the specifics and discussion of feasibility, potential complications, and hopeful outcomes. After that, due diligence is performed to ensure efficient subcellular fraction purity and subcellular proteome coverage. Once appropriate proteome coverage has been validated a decision is made on the final experimental design and cost. Protein lysates (anywhere from 25-200ug) of subcellular fractions from cell culture or animal/human tissue are subjected to in-solution enzymatic digestion to digest the protein into peptides, and the resultant peptides are then desalted. Each in-solution digest is then analyzed as an independent sample by mass spectrometry. The mass spec raw data is then processed in the quantitative proteomics software program Progenesis followed by searching against the appropriate database with the software program Mascot. The Mascot peptide and protein identification data is then imported into Progenesis. The proteins are then analyzed for expression changes across the different treatment groups. The proteomic data can then be imported into the bioinformatics program Perseus for continued analysis and data preparation.